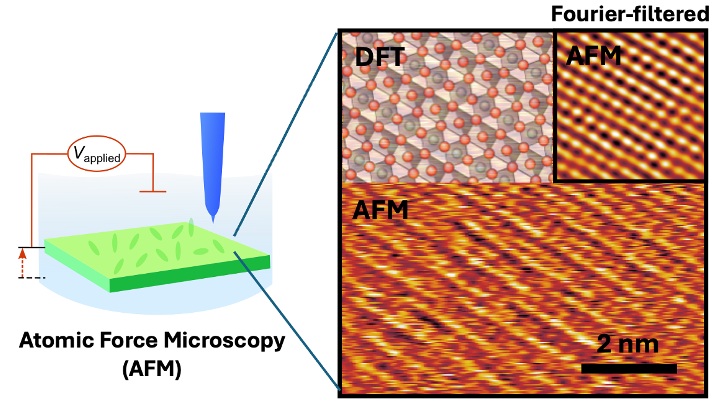
Scientific Achievement
Atomic Force Microscopy (AFM) imaging of the RuO2-water interface shows the evolution of surface water during the water oxidation reaction. This achievement is unlocked by the advancement in AFM and charged surface modeling.
Significance and Impact
Water electrolysis can provide sustainable hydrogen for energy and manufacturing. This process starts by removing hydrogen from water (“dehydrogenation”). This work allows researchers to see this process to create a precise model of electrolysis.
Research Details
A well-defined RuO2(110) surface was used as an electrode for the water oxidation experiment.
EC-AFM showed surface water undergoing dehydrogenation on the oxide with the underlying oxide structure staying intact.
Combined experimental and computational results suggest this dehydrogenation occurs with many surface water configurations.
The result suggests that water oxidation is a collective process and should not be treated as an isolated adsorption event.
Reference
Reese, A. J.; Gelin, S.; Maalouf, M.; Wadehra, N.; Zhang, L.; Hautier, G.; Schlom, D.G.; Dabo, I.; Suntivich, J.Tracking Water Dissociation on RuO2(110) Using Atomic Force Microscopy and First-Principles Simulations, J. Am, Soc. Chem., 2024, ASAP. DOI: 10.1021/jacs.4c13164
Acknowledgment of Support
This work was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award no. DE-SC0023415 (Center for Electrochemical Dynamics for Reactions on Surfaces, an Energy Frontier Research Center.) The EC-AFM capability, particularly the liquid cell, was developed through the support of the U.S. Department of Energy, Office of Science, Basic Energy Sciences Award no. DE-SC0023896. The electrochemical interface modeling capability was developed through the support of the U.S. Department of Energy, Office of Science, Basic Energy Sciences, CPIMS (Condensed Phase and Interfacial Molecular Science) Program, under Award No. DE-SC0018646. First-principles calculations were performed using Anvil at Purdue University through allocation CHM230047 from the Advanced Cyberinfrastructure Coordination Ecosystem: Services &Support (ACCESS) program, which is supported by National Science Foundation grants nos. 2138259, 2138286, 2138307,2137603, and 2138296.
Tracking Water Dissociation on RuO2(110) Using Atomic Force Microscopy and First-Principles Simulations
Center for Electrochemical Dynamics and Reactions on Surfaces (CEDARS)
CEDARS studies how electrons and protons move and how bonds form and break on the surface during the process of producing hydrogen from water. We combine precise methods for growing materials with various techniques like scattering and spectroscopy to examine the intermediate steps involved. We also use computer modeling based on fundamental principles. Our team is diverse and comes from different fields of study, including materials science, chemistry, and computational science.